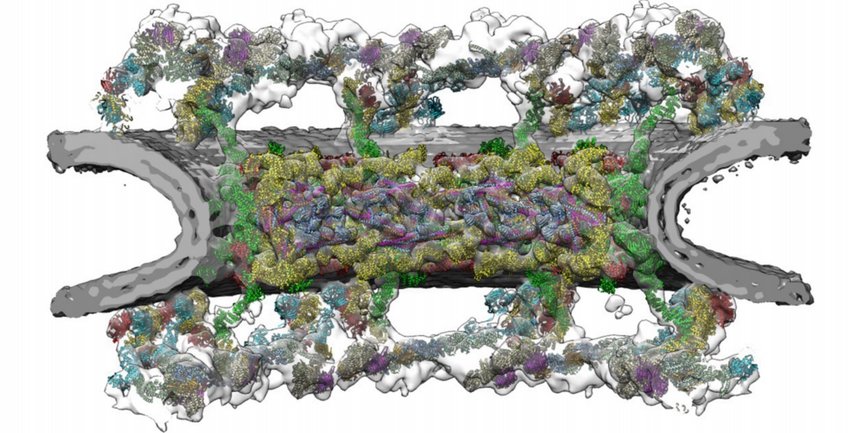
Nuclear Pore Complexes
Department of Molecular Sociology (MS)
< go back to other projects
Nuclear pore complexes (NPCs) are fundamental components of all eukaryotic cells. They are giant channels that perforate the nuclear envelope and mediate transport of macromolecules between the cytoplasm and the nucleus. In mammals, around 1000 protein building blocks assemble into an intricate cylindrical architecture that fuses the inner and outer nuclear membranes, making the NPC possibly the largest protein complex in the cell. We aim to understand how this architecture enables nucleocytoplasmic exchange in conjunction with the regulation of gene expression. In particular, we study three different aspects:
- The compositional and conformational dynamics of NPC architecture. NPCs compositionally and conformationally adapt to context-specific needs. It has become clear that there are prominent differences in nuclear pore function across cell types and organisms. Such alterations play a critical role during cell differentiation, malignant transformation and genetic diseases. The question to which extent NPCs within the same cell fulfill dedicated tasks remains an active area of research.
- The evolutionary diversity and origin of NPCs. The nuclear compartment is a hallmark of eukaryotes and therefore, the NPCs themselves are deeply rooted in the origin of eukaryotes. We have shown that NPCs are surprisingly diverse across the eukaryotic tree of life. Changes in the NPC may have left an imprint of the evolutionary history of eukaryotes that might ultimately allow to precisely root their origin.
- How cells build and degrade NPCs. Due to the complexity of their architecture, the assembly, maintenance and quality control of NPCs imposes a formidable challenge for cells. Two mechanistically distinct assembly pathways conceptualise how NPCs are made during interphase and at the end of mitosis, respectively, in higher eukaryotes. We have previously shown that an alternative modus operandi is relevant to oogenesis and that NPC turnover proceeds through selective autophagy by nuclear envelope budding. We are interested in the molecular mechanisms of NPC maintenance and structural analysis of the respective assembly and disassembly intermediates. These processes are not only relevant to development, but also to cellular homeostasis and aging.
Selected Publications
AI-based structure prediction empowers integrative structural analysis of human nuclear pores.
Science. 2022 June; 376 (6598), doi: 10.1126/science.abm9506.
Co-translational assembly orchestrates competing biogenesis pathways.
Nature Communications. 2022 March; 13:1224; doi:10.1038/s41467-022-28878-5.
Nuclear pores dilate and constrict in cellulo.
Science. 2021 Nov 11; doi:10.1126/science.abd9776.
Cone-shaped HIV-1 capsids are transported through intact nuclear pores.
Cell. 2021 Feb 10; 184, 1-15. doi:10.1016/j.cell.2021.01.025.
In cell architecture of the nuclear pore complex and snapshots of its turnover.
Nature. 2020 Sept 02; 586, 796-800. doi: 10.1038/s41586-020-2670-5.
Structure and Assembly of the Nuclear Pore Complex.
Annu Rev Biophys. 2019 May 6;48:515-536. doi: 10.1146/annurev-biophys-052118-115308.
In situ architecture of the algal nuclear pore complex.
Nat Commun. 2018 Jun 18;9(1):2361. doi: 10.1038/s41467-018-04739-y.
Postmitotic nuclear pore assembly proceeds by radial dilation of small membrane openings.
Nat Struct Mol Biol. 2018 Jan;25(1):21-28. doi: 10.1038/s41594-017-0001-9.
From the resolution revolution to evolution: structural insights into the evolutionary relationships between vesicle coats and the nuclear pore.
Curr Opin Struct Biol. 2018 Oct;52:32-40. doi: 10.1016/j.sbi.2018.07.012.
Molecular architecture of the inner ring scaffold of the human nuclear pore complex.
Science. 2016 Apr 15;352(6283):363-5. doi: 10.1126/science.aaf0643.
Nuclear pore assembly proceeds by an inside-out extrusion of the nuclear envelope.
Elife. 2016 Sep 15;5. pii: e19071. doi: 10.7554/eLife.19071.
In situ structural analysis of the human nuclear pore complex.
Nature. 2015 Oct 1;526(7571):140-143. doi: 10.1038/nature15381.
Integrated structural analysis of the human nuclear pore complex scaffold.
Cell. 2013 Dec 5;155(6):1233-43. doi: 10.1016/j.cell.2013.10.055.
Cell type-specific nuclear pores: a case in point for context-dependent stoichiometry of molecular machines.
Mol Syst Biol. 2013;9:648. doi: 10.1038/msb.2013.4.