Florian Wilfling Research Group Projects
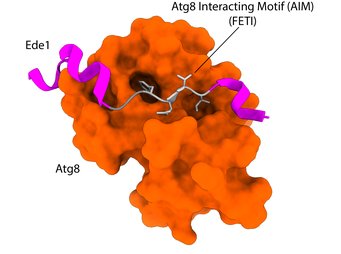
Intrinsic Autophagy Receptors
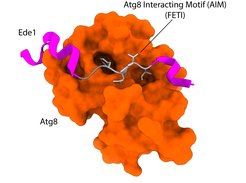
Macromolecular complexes (MC) are cellular machines that perform a wide array of vital tasks. They operate in a controlled, coordinated fashion within the crowded environment of the cell and, due to their essential roles, accumulation of dysfunctional MCs leads to age-related diseases. Despite recent technological advancements, mechanisms responsible for detection and removal of excess or dysfunctional MCs remain elusive. Recently, we uncovered a role of selective autophagy in degradation of the nuclear pore complex and the clathrin-mediated endocytosis machinery which is dependent on the action of intrinsic autophagy receptors. Intrinsic receptors represent functional subunits of a given macromolecular machine but can, if needed, recruit the autophagy machinery to engulf and degrade the respective complex. As such, intrinsic receptors provide an inbuilt quality control function that monitors the assembly state and/or functionality of macromolecular machines.
We are interested in systemically mapping and defining the intrinsic receptor landscape in different species as well as understanding how these receptors distinguish the functional from the degradation-prone state of a macromolecular complex.
Further reading:
Cargo Arrangement for Autophagic Degradation

Macroautophagy can encapsulate a large set of different cargoes, ranging from membrane-bound organelles in mitophagy or ER-phagy to encapsulated pathogenic microbes in xenophagy or, in the case of proteins, either specific structural assemblies (e.g. Cvt pathway, proteophagy or ferritinophagy) or assemblies with particular material properties (aggrephagy). In many cases, the organisation of the autophagic cargo prior to degradation is poorly understood on a structural level. Therefore, we combine structural proteomics with advanced light microscopy and in situ cryo-electron tomography to directly determine and visualize the composition of autophagic cargo degraded under various different conditions.
Further reading:
Autophagosome Biogenesis

During macroautophagy, autophagosomes are synthesized de novo to engulf and transport cytosolic material for lysosomal degradation. The targeted material can be extremely diverse in size and nature, ranging from proteins to organelles to invading pathogens, such as bacteria. How autophagosomes are built to enable uptake of such diverse cargo is still incompletely understood. We use correlative cryo-electron tomography to get insights into how autophagic cargo initiates and influences autophagosome biogenesis on a structural level. In a first study, we have used the model organism S. cerevisiae to reveal transient autophagy intermediates at high-resolution directly in situ during bulk autophagy, which highlights both the structural features of phagophores and their organelle interactome at different stages during autophagosome biogenesis. We will use and develop this method further to understand how cargo influences phagophore initiation and maturation on a structural level and how the autophagic biogenesis machinery shapes the membrane during this process. Visualising rare and transient cellular events, such as those which occur during autophagosome biogenesis, is challenging using conventional cryo-electron tomography sample preparation methods. Therefore, to overcome these limitations, we, together with the group of Jürgen Plitzko at the MPI of Biochemistry, are currently developing new methods to freeze biological samples for correlative cryo-ET.
Further reading: