Structure of serotonin receptor in presence of lipids resolved
The lipid bilayer stabilises the receptor in a more tightly packed state
Once again, a step has been taken towards clarifying the action of the neurotransmitter serotonin, often referred to as the "happiness hormone", to one of its receptors, 5-HT3AR. An international team of researchers has succeeded now in elucidating the structure of this serotonin receptor directly in the lipid layer. Serotonin binds to this receptor outside the cell, which leads to the opening of a specific ion pore in the membrane part of the receptor.
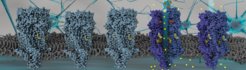
The 5-HT3A receptor (5-HT3AR) is a pentameric ligand-gated ion channel (pLGIC) which regulates fast neurotransmission in brain. When cations flow through the inner channel of the receptor physiological response is the result.
Decades of studies demonstrated the role of lipids to play in modulation pLGIC function, however, the details underlying the molecular mechanism are still not well understood. 5-HT3AR represents an interesting case as despite several structures of it have been reported in detergents in both presence and absence of its agonist serotonin. But the mechanism of 5-HT3AR gating remains an open question, in part due to the lack of an
unambiguous open-state receptor structure. Importantly, detergents typically used for purification of membrane proteins from membranes remove the functionally important lipids which may affect the conformation of the proteins and thus their functions.
In the now published paper in Nature Communications, Yingyi Zhang and Patricia Dijkman from the Max Planck Institute of Biophysics and colleagues address this lack of mechanistic understanding of lipid regulation of the receptor by providing a structural basis for the modulation of 5-HT3AR conformation and gating by the lipid environment. The authors solved the structure of the receptor reconstituted in lipid bilayer nanoparticles in the presence and absence of serotonin using cryo-electron microscopy.
In collaboration with the group of Shuguang Yuan from the Chinese Academy of Sciences, Shenzhen, China, the researchers could show by using molecular dynamics simulations that the lipid-embedded receptor structure in the serotonin-bound form represents an open channel. This channel allows free diffusion of sodium ions across the membrane region. The agonist-bound structure in a lipid bilayer shows a much larger pore opening than any of the previously reported agonist-bound structures in detergent.
Structural details of the serotonin receptor
Furthermore, these lipid-embedded structures show substantial differences compared to detergent-based structures and provide a framework to explain receptor gating. The leading author, Misha Kudryashev from the Max Planck Institute of Biophysics highlights the importance of native context: “In the lipid bilayer, the outer transmembrane helices show a displacement of more than 10 Å compared to their position in detergent. We are now able to propose a mechanism by which the membrane environment modulates the structure and function of 5-HT3AR. Importantly, due to the highly conserved residues of related receptors involved in the proposed mechanism of lipid modulation, we believe that our findings can be generalised to other pLGICs."
5-HT3AR is of high pharmaceutical relevance as a drug target for the treatment of, for example, emesis and irritable bowel disease.
