Aerobic Terminal Oxidases
Project Group of Department of Molecular Membrane Biology (MMB)
Terminal oxidases are the terminal enzymes of respiratory chains which transfer electrons from donors to a final acceptor. The respiratory chains of aerobic organisms use molecular oxygen as the final electron acceptor. The respiratory chain of mitochondria is most well known. Its terminal oxidase, called complex IV or cytochrome c oxidase, is a canonical member of the superfamily of the heme- and copper-containing terminal oxidases. Aerobic terminal oxidases generate an electrochemical proton potential across the membrane, because electrons are delivered from the outer surface of mitochondria or of aerobic bacteria, whereas protons consumed in water formation originate from the inner surface. The terminal oxidases of the heme-copper superfamily in addition pump protons across the membrane. There is a general consensus that four additional protons are pumped by the canonical oxidases in addition to the four protons consumed per dioxygen molecule.
The superfamily can be divided into three families. Family A contains the canonical oxidases like the mitochondrial cytochrome c oxidases or those from many proteobacteria, including some quinol oxidases like cytochrome bo from Escherichia coli. Family B mainly consists of archaeal and some bacterial oxidases, and family C of the cbb3 type cytochrome c oxidases. These latter ones are only found in bacteria, they are essential for nitrogen fixation and the colonisation of host tissues by pathogenic bacteria.
The terminal oxidases of the cytochrome bd type found in bacteria and in some archaea are completely unrelated to the heme–copper oxidases and do not pump protons.
Terminal oxidases of all four types/families are under investigation in the department.
Canonical cytochrome c oxidases
Cytochrome c oxidases use cytochrome c as the electron donor to reduce molecular oxygen. Accordingly they catalyse the following reaction:
4 cyt c+II + O2 + 8 H+(i) → 4 cyt c+III + 2 H2O + 4 H+(o)
Four electrons are provided from cytochrome c molecules. Four protons are consumed upon formation of two water molecules and four protons are pumped. All protons originate from the intracellular side of the bacterium or mitochondrion. The resulting electrochemical potential can be used to synthesise ATP by the ATP-synthase or to drive secondary active transport.
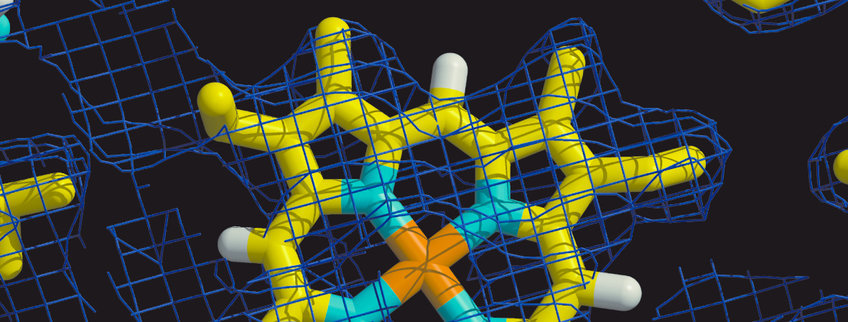
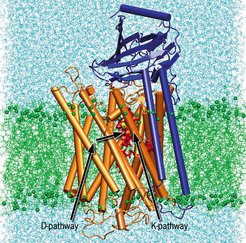
Having determined the first structure of a aa3-type cytochrome c oxidase (see figure), a 4-subunit protein of the soil bacterium Paracoccus denitrificans, we currently aim to understand the mechanism of action of this important enzyme. Its active site is formed by heme a3 and a copper atom (CuB). Oxygen is bound to the heme a3 iron of the doubly reduced active site. The canonical cytochrome c oxidases possess two proton transfer pathways (see figure), the so called K-pathway leading directly to the active site, and the D-pathway leading to a glutamyl residue (E278). The further pathway is not obvious, but there must be a possibility for proton transfer to the active site, and to the heme propionate area. All pumped protons appear to use the D-pathway, only one or two protons accompanying the initial reduction of the oxidized cytochrome c oxidase are taken up via the K-pathway.
The questions to be answered are:
- Which of the four electron transfers (from cytochrome c to the active site) are coupled to proton pumping?
- How are protons assigned to be substrate or pumped protons?
- What is the precise structure of the catalytic intermediates?
- Is there a peroxide bridge in the active centre in the oxidised state?
- Where is the proton exit pathway?
We address these questions by site-directed mutagenesis, UV/Vis-spectroscopy, EPR-spectroscopy (together with Dr. Fraser MacMillan from the university of East Anglia in Norwich, U.K.), atomic absorption spectroscopy (together with the department of Biophysical Chemistry), generation of intermediates of the catalytic cycle, injection of single electrons combined with kinetic measurements (stopped-flow measurements) and x-ray crystallography of intermediate states and variants of the enzyme.
These experimental approaches are complemented by theoretical work, namely electrostatic calculations and molecular dynamics simulations.
It has become evident, that, in contrast to the previous belief, protons are also pumped during the reduction of the enzyme prior to its reaction with oxygen. The mechanism of proton pumping remains to be elucidated.
Other terminal oxidases
We try to crystallize selected members of the other classes/types of terminal oxidases. We recently succeeded to determine the structure of the cbb3 type cytochrome c oxidase from Pseudomonas stutzeri.
