Schara Safarian: Molecular Mechanisms of Pathogen Respiration
How do pathogens manage their bioenergetics upon host infection? What can we learn from these processes for the development of novel antibiotics?
Cellular respiration is one of the most fundamental metabolic processes of life. In eukaryotes, this process in facilitated by distinct and highly conserved respiratory chain complexes. In contrast, bacteria are equipped with a variable set of respiratory chain enzymes which enable to maintain aerobic respiration under extreme environmental conditions. Cytochrome bd is a terminal oxygen reductase that catalyzes the reduction of molecular oxygen to water. It is evolutionarily unrelated to the heme-copper oxidase (HCO) superfamily that includes the human cytochrome c oxidase. While members of the HCO superfamily are conserved throughout all domains of life, cytochrome bd-type oxidases are exclusively found in prokaryotes. Most pathogenic bacteria, particularly M. tuberculosis, rely on cytochrome bd for infection of the human host and for the transition for acute to chronic states of disease.
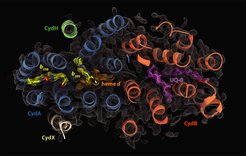
The project group of Dr. Schara Safarian at the department of Molecular Membrane Biology studies the mechanism of cytochrome bd action on the atomic level by employing biochemical, biophysical, and computational methods. Gaining insights into the molecular framework of proteins is a fundamental requirement to understand function. Thus, a key focus of the Safarian lab is the determination of cytochrome bd structures by single-particle electron cryo-microscopy and X-ray crystallography. In conjunction with orthogonal methods such as spectroscopy, or hydrogen-deuterium exchange mass spectrometry, and molecular dynamics simulations we are able to dissect the molecular complexity of oxygen reduction. In addition, we run a screening platform for the identification of novel cytochrome bd inhibitors that can qualify as lead molecules for drug development.
Beyond delineating the precise function of terminal oxidases, we aim to understand upstream physiological processes related to assembly and maturation of metalloproteins. Knowledge about the mechanism of heme insertion remains mostly unknown for membrane-integrated and soluble cytochromes but is urgently required for the development of antimicrobial drugs targeting the energy metabolism and respiratory re-wiring of bacteria. In this context, we study the fundamental process of bacterial heme transport facilitated by the ABC transporter CydDC across the cytoplasmic membrane which is a pre-requisite for the assembly of terminal oxidases and c-type cytochromes.
Project Group Members
Tsai-Hsuan Weng, Di Wu.
Alumnis: Melanie Radloff, Tamara Grund, Anna Franziska Finke, Roan Groh
