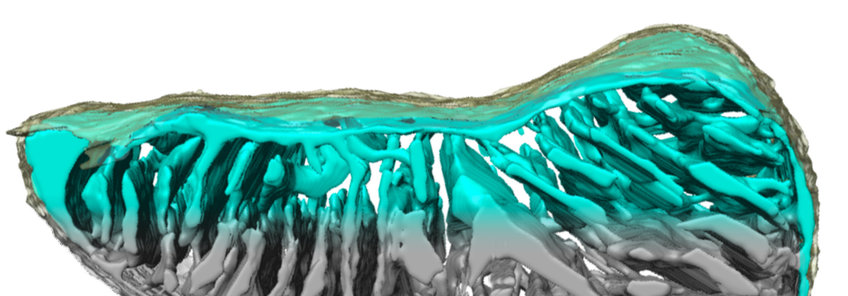
CryoET of mitochondria
CryoET makes it possible to not only examine mitochondrial membranes in molecular detail, but also whole mitochondria to study overall cristae organization and cristae junctions (Bohnert et al., Cell Metabolism 2015), age-related changes in mitochondrial membrane structure, or mitochondrial fusion. The resolution obtained with whole organelles tends to be lower, because the increased sample thickness reduces image contrast.
CryoET of active protein translocases in whole mitochondria
We developed a technique to label active protein translocases in mitochondria by electron cryo-tomography by marking them with quantum dots. In this way we were able to visualize the three-dimensional distribution and organization of protein import sites in mitochondria. We have shown that import sites cluster together in the vicinity of cristae junctions. Subtomogram averaging revealed new details of the mitochondrial protein import machinery in action. Our approach can be used for site-specific labelling of a multitude of membrane proteins by electron cryo-tomography in the future (Gold et al., Nat Comm 2014).
Changes in mitochondrial inner membrane structure during ageing
We have shown by cryoET that the ATP synthase dimer rows, and the dimers themselves, dissociate in ageing Podospora anserina, a model organism with a 20-day lifespan used to study senescence (Daum et al., PNAS 2013). If our hypothesis is right and the cristae are micro-compartments that are required for efficient ATP production, senescent mitochondria may not be able to satisfy the high energy demand of fully functioning cells.
CryoET of mitochondrial outer membrane fusion
In collaboration with Mickael Cohen at the IBPC in Paris, we have used cryoET to examine mitochondrial outer membrane fusion. Fusion is crucial for proper mitochondrial function and involves large GTPases called mitofusins. The discrete steps that allow mitochondria to attach to one another and merge their outer membranes were unknown. By combining an in vitro mitochondrial fusion assay with cryoET, we visualized the junction between isolated S. cerevisiae mitochondria and observed complexes that mediate this attachment. We found that cycles of GTP hydrolysis induce progressive formation of a docking ring around extended areas of contact. Further cycles of GTP hydrolysis trigger local outer membrane fusion at the periphery of the contact region. Our findings unravel key features of mitofusin-dependent fusion of outer membranes and constitute an important advance in our understanding of how mitochondria connect and merge (Brandt et al., eLife 2016).