Ana J. García-Sáez Department Projects
Mitochondrial Apoptosis
Regulated Necrosis
Mitochondrial Apoptosis
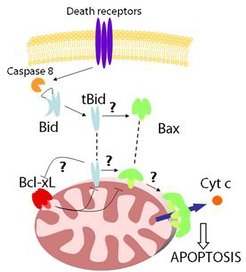
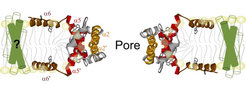
The permeabilization of the mitochondrial outer membrane (MOM) is a key step in apoptosis that leads to the release of the so-called apoptotic factors, like cytochrome c, into the cytosol, thereby inducing the activation of caspases and cell death. This process is controlled by the proteins of the BCL-2 family, which form a complex interaction network whose outcome decides the cell fate. They are classified into three subgroups:
i) antiapoptotic proteins, like Bcl-2 or Bcl-xL;
ii) proapoptotic proteins like Bax or Bak that directly mediate MOM permeabilization;
iii) and the BH3-only proteins, including Bid or Bim, which act like sensors for the different apoptotic stimuli and initiate apoptosis.
Since the BCL-2 proteins have an important role in tumorigenesis and in the cellular responses to anti-cancer therapies, understanding their functioning is of great therapeutic interest. In our group, we study the stoichiometry and interaction preferences of Bcl-2 proteins complexes by exploiting the single molecule toolbox implemented in the lab. We also aim to define the composition, dynamics and structure of apoptotic foci and to understand how they are integrated to orchestrate function. In addition, we are interested in understanding the mechanisms underlying mitochondrial dynamics and the contacts of mitochondria with other cellular membranes.
Composition, Structure and Dynamics of Apoptotic Foci
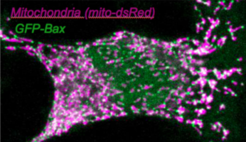
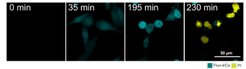
In previous work, we and others found that Bax and Bak protein dimers assemble at specific apoptotic foci to mediate MOMP. This EU-funded project aims to define the composition, dynamics and structure of apoptotic foci to elucidate their integral role in apoptosis regulation. The multidisciplinary approach promises to solve the question of how Bax and Bak mediate MOMP and to help develop apoptosis modulators for medical applications.
Effect of BCL-2 Proteins on Mitochondria Structure and Function
Besides their role in apoptosis, the proteins of the BCL-2 family have additional functions on several mitochondrial processes, which, together with the molecular mechanisms involved, remain poorly understood. In this project, we aim to dissect the differential role of BCL-2 proteins in the regulation of not only of mitochondrial shape and ultrastructure, but also of metabolism, calcium signaling and ROS, and to uncover the underlying molecular mechanisms.
Regulated Necrosis
Beyond apoptosis, it has been recently recognized that several forms of necrotic cell death are also regulated by genetically encoded signaling pathways that we are only beginning to understand. Common to the execution of necroptosis, pyroptosis and ferroptosis is the permeabilization of the plasma membrane that releases the cellular contents to the microenvironment and initiates immune responses.
A second line of research in the group focuses on other forms of regulated cell death. We have shown that both necroptosis and ferroptosis execution involve the formation of plasma membrane nanopores that can be blocked by osmoprotection (Ros et al. Cell Reports, 2017; Pedrera et al., CDD, 2020). Membrane pore formation is emerging as a common theme in regulated forms of regulated cell death, including new pathways like necroptosis, pyroptosis or ferroptosis, which we are studying with similar strategies.
Necroptosis
Regulation of MLKL in necroptosis
Necroptosis is a newly discovered form of regulated necrosis that is inflammatory and plays a role in a number of diseases associated with chronic inflammation and infection. It results in release of the cellular contents after plasma membrane permeabilization dependent on the pseudokinase mixed lineage kinase domain-like (MLKL) protein, which is the most terminal effector of necroptosis known to date. Additionally, non-deadly roles of MLKL in intracellular vesicle trafficking that seem to counterbalance necroptotic cell death have been recently described. However, the molecular mechanism behind these MLKL functions remains obscure and is a matter of debate.
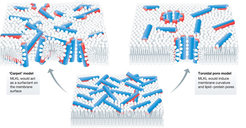
The main goal of this project is to shed new light on the molecular mechanism how MLKL executes necroptosis. To this aim, we will uncover the structural elements that regulate MLKL activity and determine the contribution of the different MLKL isoforms to MLKL deadly and non-deadly functions.
This multidisciplinary project combines cell biology and advanced microscopy with molecular dynamic simulations to disclose new molecular steps involved in the coordination of necroptosis.
Mechanisms of MLKL in plants and animals
The discovery of a new protein family in seed plants that is structurally and functionally homologous to animal MLKL suggested that similar mechanisms may take place in the execution of cell death in plants. This project aims to understand the molecular principles of MLKL-mediated cell death and immunity by determining and comparing the nanoscale organization and cellular dynamics of this protein family in plant and animal cells.
Ferroptosis
Membrane alterations in ferroptosis
Ferroptosis is a caspase-independent form of regulated necrosis characterized by the generation of iron-dependent lipid peroxides in cellular membranes. While it is still unknown how lipid peroxidation leads to ferroptotic cell lysis and death, plasma membrane rupture releases pro-inflammatory damage-associated molecular patterns (DAMPs) leading to necroinflammation and activation of the innate immune system.
The overarching goal of this project is to understand how lipid peroxidation triggers plasma membrane permeabilization leading to cell death. Building on our expertise on characterizing membrane permeabilization mechanisms in regulated cell death, we aim to determine the alterations in the biophysical properties of cellular membranes in ferroptotic cells. We use advanced microscopy and biophysical tools to examine the changes in their permeability, fluidity and lateral organization, transmembrane asymmetry and mechanical properties. Futhermore, we use lipidomic analysis to determine which peroxidized lipid species and derivatives are generated in subcellular membranes during ferroptotic progression.